1) A. baumannii response to stress
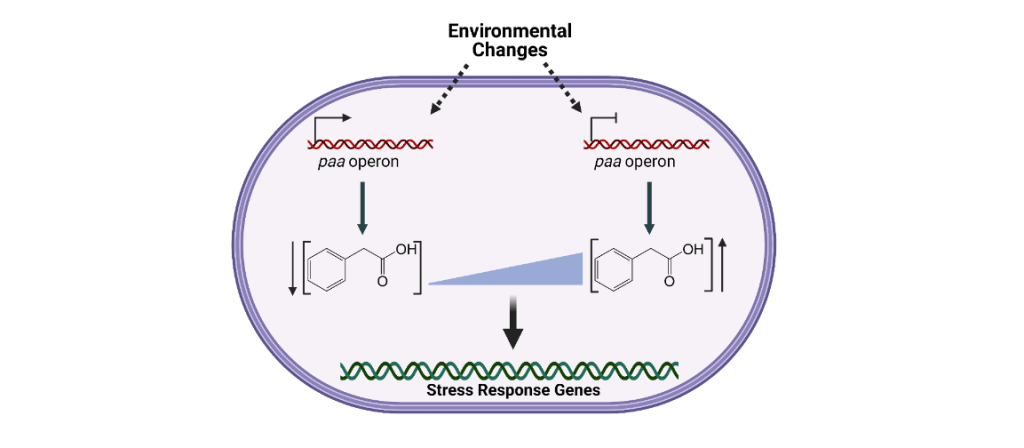
- Phenylacetic acid (PAA) catabolism and A. baumannii response to stress
Pathogenic bacteria are exposed to an array of stressful conditions during infection. As such, the ability of pathogens to respond and adapt to stress is essential to their capacity to successfully cause disease. Understanding the mechanisms employed by A. baumannii to sense and respond to stress has a remarkable therapeutic potential, as interfering with these processes may result in novel therapeutic strategies to combat infections. Multiple studies in A. baumannii have shown that different stress conditions lead to drastic changes in the expression of the paa operon. The paa operon encodes the enzymes required to degrade phenylacetic acid, an intermediate metabolite of phenylalanine catabolism. We have established that in the presence of sub-inhibitory concentrations of different antibiotics, A. baumannii dramatically increases the transcription of the paa operon (Hooppaw Anna J et al., 2022). Conversely, this operon is repressed by the addition of hydrogen peroxide. Furthermore, exogenous addition of PAA or its non-metabolizable derivative 4-Fluoro-PAA (4F-PAA) reverts these phenotypes, supporting the signaling capacity of PAA. Importantly, we found that PAA catabolism mutants in multiple Acinetobacter strains are more sensitive to oxidative stress and antibiotics. We hypothesize that Acinetobacter employs a previously unrecognized signaling pathway in which PAA acts as a homeostatic signaling molecule. High or low PAA concentrations are sensed by the cell, triggering various stress responses.
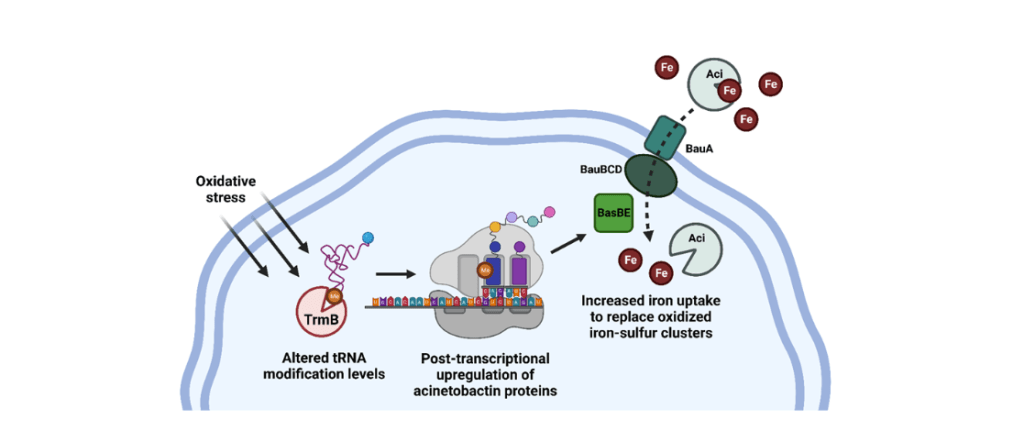
- tRNA methylation and A. baumannii response to stress
Recent studies have begun to explore the importance of tRNA methylation in the regulation of bacterial stress responses; however, tRNA methyltransferases (trm) have not been investigated in A. baumannii. Our lab is currently exploring how a variety of tRNA methyltransferases can post-transcriptionally mediate a multitude of Ab stress responses, including oxidative stress, antibiotics, pH stress, osmotic stress, and more. In a recent paper, we demonstrate that the m7G methyltransferase TrmB is crucial for a particularly virulent, colistin-resistant strain of A. baumannii to respond to stressors encountered during infection, and that loss of TrmB dramatically attenuates a murine pulmonary infection. Given the current efforts to use another trm, TrmD, as an antimicrobial therapeutic target, we propose that TrmB, and other tRNA methyltransferases, may also be viable options for drug development to combat multi-drug resistant A. baumannii (Mc Guffey et al, mBio, 2023).
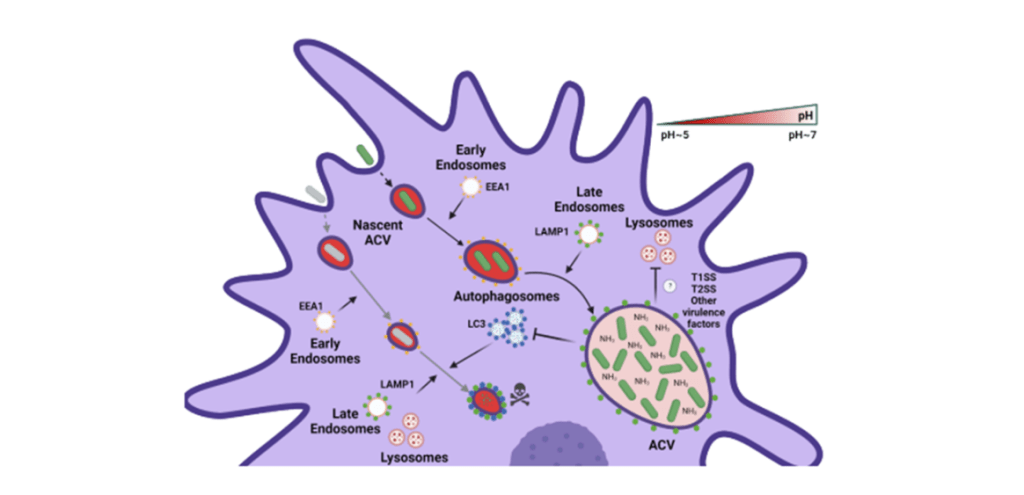
2) A. baumannii replication inside of macrophages
Respiratory tract infections are the principal disease associated with A. baumannii. Alveolar macrophages play a critical role in overcoming respiratory infections caused by this pathogen. Recently, we and others have shown that new clinical isolates of A. baumannii, but not the common lab strain ATCC 19606 (19606), can persist and replicate in macrophages within spacious vacuoles that we called Acinetobacter Containing Vacuoles (ACV – Sycz et al., PLOS pathogen, 2021). We show that the modern A. baumannii clinical isolate 398, but not the lab strain 19606, can infect alveolar macrophages and produce ACVs in vivo in a murine pneumonia model. Both strains initially interact with the alveolar macrophage endocytic pathway, as indicated by EEA1 and LAMP1 markers; however, the fate of these strains diverges at a later stage. While 19606 is eliminated in an autophagy pathway, 398 replicates in ACVs and are not degraded. We show that 398 reverts the natural acidification of the phagosome by secreting large amounts of ammonia, a by-product of amino acid catabolism. We propose that this ability to survive within macrophages may be critical for the persistence of clinical A. baumannii isolates in the lung during a respiratory infection (Distel et al, PLOS pathogen, 2023). Even though ammonia production is necessary for the initial establishment of the ACVs, A. baumannii may use other virulence factors to optimize the nutrient content and block the host endosomal pathway, we are currently investigating this hypothesis.
3) Developing novel models for A. baumannii pulmonary pathogenesis
The most common disease caused by Acinetobacter baumannii is pneumonia. As A. baumannii isolates are becoming increasingly more drug-resistant, even treated infections can persist for weeks to months or until the patient dies. Despite this, the most common A. baumannii murine pulmonary infection models are acute 24-36 hour infections with high, unnatural inoculums. Thus, this model fails to recapitulate clinical infection. One focus of our research is therefore to develop long-term infection models to identify bacterial factors required for virulence and persistence. Additionally, as one of the most common forms of pneumonia caused by A. baumannii is ventilator-associated pneumonia (VAP), we are also aiming to develop murine models of infection to simulate such infections.
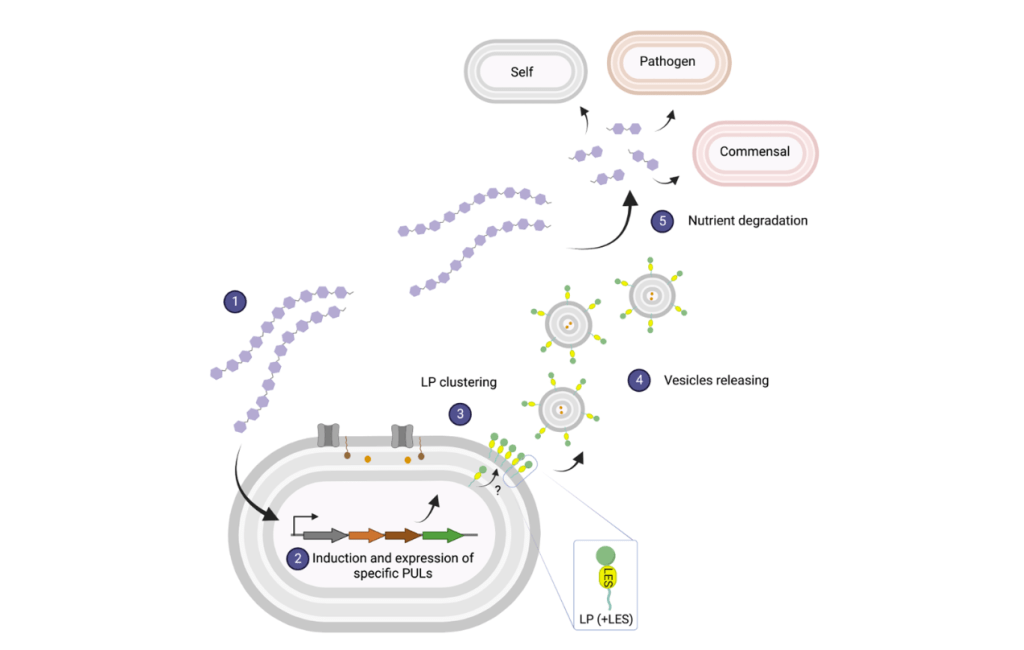
4) OMV biogenesis in B. thetaiotaomicron
Bacteroides spp. is a prominent member of the human gut microbiota known to produce large quantities of uniformly-sized Outer Membrane Vesicles (OMVs). It has been reported that OMVs actively contribute to gut homeostasis through their immunomodulatory properties, and due to their ability to degrade a wide array of dietary and host polysaccharides. Despite their physiological relevance, no mechanism for OMV biogenesis has been established. OMV biology remains one of the least studied fundamental processes in microbiology.
We previously demonstrated that, in vitro, Bacteroides fragilis (Bf) and Bacteroides thetaiotaomicron (Bt) OMVs are enriched with glycosidases, most of them being lipoproteins encoded within polysaccharide utilization loci (PULs). Proteomic analyses of Bt OMVs produced in the presence of different glycan nutrients showed that Bt specifically alters the profile of glycosidases packaged into OMVs to optimize the degradation of the available polysaccharides. We demonstrated that sorting enzymatic cargo into OMVs relies on a negatively charged N-terminal signal, located next to the lipobox, known as lipoprotein export signal (LES). Our preliminary data indicates that, in Bacteroides spp., OMV are the result of a highly orchestrated physiological process (Valguarnera et al., mSphere, 2018).
By fusing fluorescent proteins to OMV-targeted and OM-retained proteins, we visualized OMV production in live cells, enabling the distinction between bona-fide vesicles and lysis byproducts in vitro (Sartorio et al., PNAS, 2023). To begin characterizing the OMV biogenesis machinery, we performed a transposon mutagenesis screening employing OMV-specific proteins as reporters. With the tools we have developed so far, in the Feldman Lab we aim to investigate the mechanism and roles of OMV biogenesis in Bt.